Insys Therapeutics

INSYS Therapeutics was a commercial-stage specialty pharmaceutical company that developed and commercialized innovative supportive care products.
They had two marketed products; SUBSYS, a proprietary sublingual fentanyl spray for breakthrough cancer pain and their Dronabinol SG Capsule, an improved second-line treatment for chemotherapy-induced nausea, CINV, and anorexia associated with weight loss in patients with AIDS. Their lead pipeline product candidate was Dronabinol Oral Solution, a proprietary orally-administered liquid formulation of Dronabinol.
These products require high Cleanroom standards for processing and packaging which Studio8 was able to accomplish with Federal Standard 209E Class 10,000 (ISO7) Cleanroom.
We upgraded the front façade with a refreshed design that includes reglazed windows, a newly painted tilt wall, an ACM canopy, and a board-formed concrete entry wall. The enhancements were completed with updated signage and the addition of flagpoles for a polished and welcoming appearance.


We modernized the canopy design by removing bulky stucco column wraps and painting the existing tube steel for a sleek, updated look.

Sublingual Lab

Compounding Pharmacy
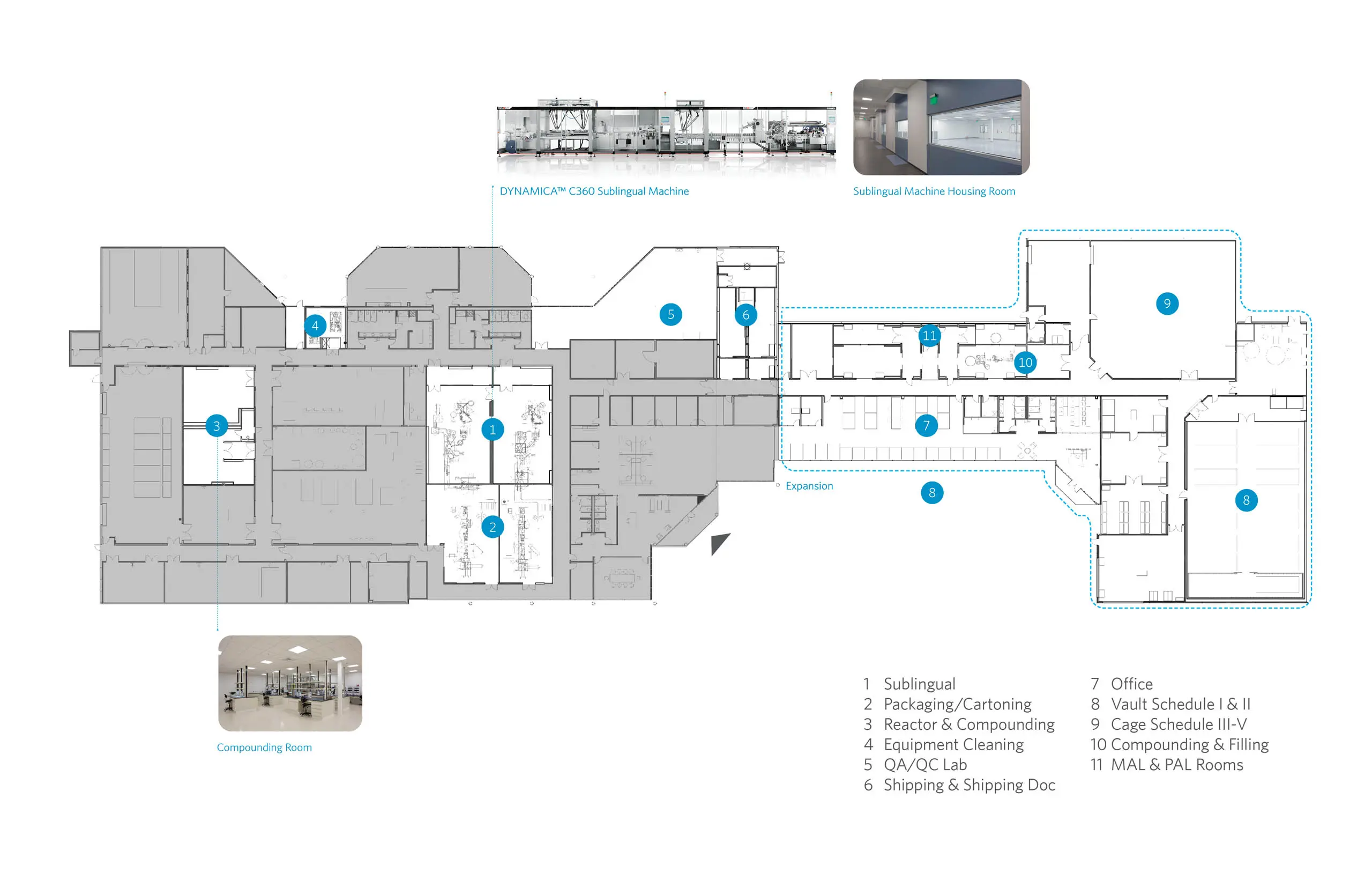
DYNAMICA™ C360, IMA Safe’s latest thermoformer, sets trends with its superior flexibility for deep draw packaging, adaptable to various production applications and modular configurations. IMA’s design team in Italy held multiple iteration meetings to refine the Sublingual filling room’s requirements, leading to the integration of an adjacent packaging/cartooning room. Weekly updates during the design development stage enabled adjustments to the architecture, MEP infrastructure, and equipment block, including the integration of a pass-through between the clean filling room and the non-clean packaging/cartooning room.

Related Projects


HID Global World Headquarters
Science & Technology, Industrial/Warehouse & Distribution

Ascension Rx Pharmacy Services Center
Science & Technology, Industrial/Warehouse & Distribution